Business Technology & Software Consultation Services for Regulated Industries
Strategic consulting and turnkey remediation delivered through the ValidKeep™ platform.
Avoid the next FDA warning letter

Why unvalidated spreadsheet logic remains one of the highest audit risks in regulated environments.
Quality Directors, Lab Managers, and Supply Chain Directors face a constant tension: processes too complex for paper, but too niche for ERP systems. The result? Uncontrolled spreadsheets running critical GxP operations. These spreadsheets handle Batch Release, Temperature Excursion management, Line Clearance, and Vendor Scorecards. They lack audit trails, electronic signatures, and change control. They're a regulatory risk waiting to become a 483 observation or Warning Letter.
We don't replace existing ERP systems. We address the Shadow IT that exists between paper and enterprise software, the processes that need validation but don't justify custom ERP modules.
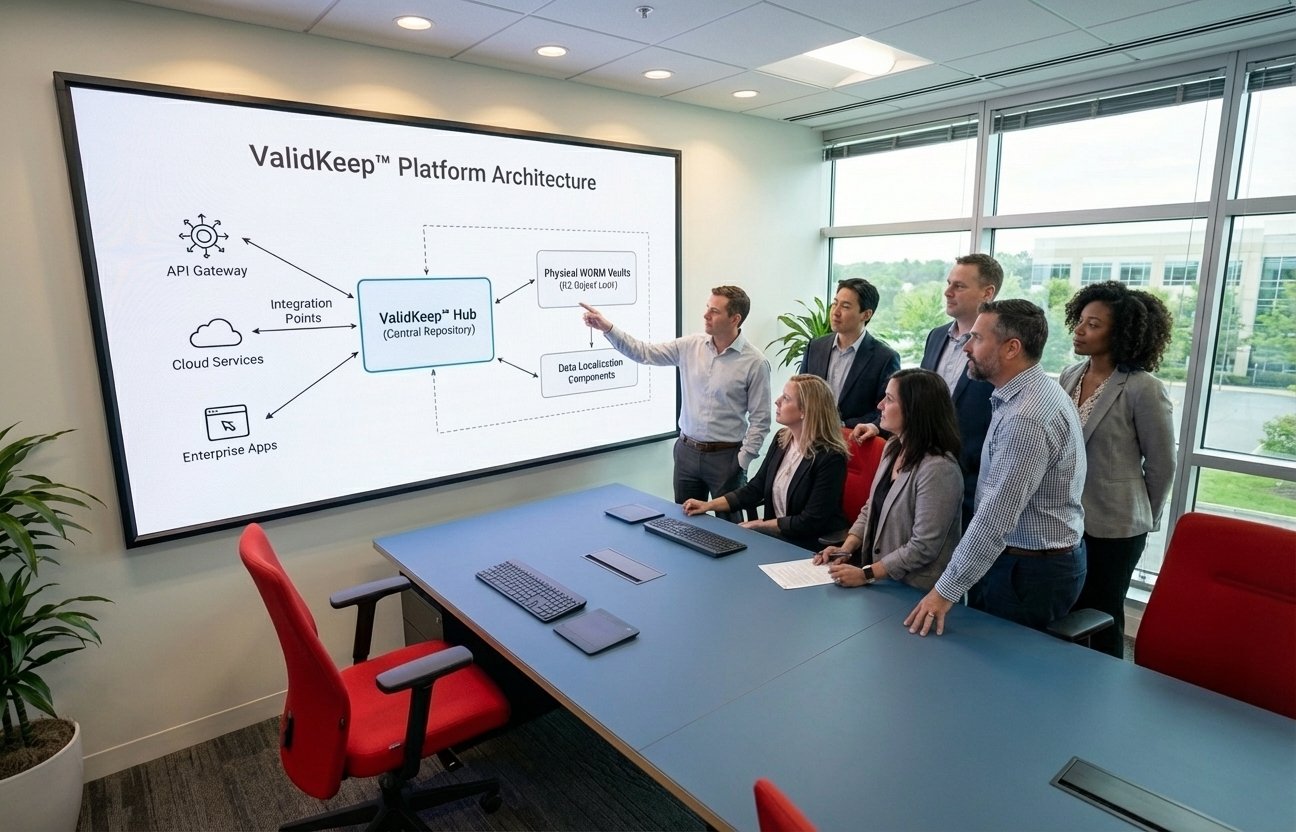
Delivery Platform

A proprietary validation and change-control platform developed by Yttrigen to support regulated remediation engagements.
Our consulting services are delivered through the ValidKeep™ platform, which provides centralized change control, documentation, and evidence repository capabilities. This platform enables our consultants to deliver validated systems with complete audit trails and compliance documentation.